Check out my first novel, midnight's simulacra!
Ergot: Difference between revisions
| (106 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[File:Ergoline.png|right|thumb|The ergoline skeleton looks not entirely unlike a cock-n-balls.]] | [[File:Ergoline.png|right|thumb|The ergoline skeleton looks not entirely unlike a cock-n-balls.]] | ||
Ergot is pronounced ur-git, '''not''' er-go. Highly toxic, it ought not be consumed. Properly modified, it has many uses, primarily due to fortuitous similarity between the ergoline skeleton and the monoamide neurotransmitters (e.g. serotonin, dopamine, | Ergot is pronounced ur-git, '''not''' er-go. Highly toxic, it ought not be consumed. Properly modified, it has many uses, primarily due to fortuitous similarity between the ergoline skeleton and the monoamide neurotransmitters (e.g. serotonin, dopamine, noradrenaline)... | ||
Ergot alkaloids ergonovine and ergotamine (and their salts) are both original List I controlled precursors under the [https://en.wikipedia.org/wiki/Controlled_Substances_Act 1970 Controlled Substances Act]. Ergocristine was added to List I in 2010 (see the DEA's 2010-03 ''[https://www.erowid.org/library/periodicals/microgram/microgram_2010-03.pdf Microgram Journal]''). Derivatives lysergic acid and its amide ("ergine") are Schedule III controlled substances under the CSA, and ergine is a Class A precursor in the United Kingdom. Subsequent derivative lysergic acid diethylamide is an original Schedule I controlled substance, and its functional analogues (any chemical "substantially similar", as if that had any kind of precise meaning) are treated as Schedule I due to the [https://en.wikipedia.org/wiki/Federal_Analogue_Act 1986 Federal Analogue Act]. Ergotamine, lysergic acid, and ergometrine (ergonovine) are all on Table I of the INCB Red List, and are thus EU Category 1 precursors. So there's clearly something worth knowing about ergot! | |||
==Some basic terminology== | ==Some basic terminology== | ||
* '''Ergot''': fungi of the genus Claviceps. All Claviceps species are ergot. The most well-known member is Claviceps purpurea (Latin ''purpuro'', purple, "to adorn/beautify"), the rye ergot fungus, which is parasitic on grasses and cereals (especially rye, Secale cereale). | * '''Ergot''': fungi of the genus Claviceps. All Claviceps species are ergot. The most well-known member is Claviceps purpurea (Latin ''purpuro'', purple, "to adorn/beautify"), the rye ergot fungus, which is parasitic on grasses and cereals (especially rye, Secale cereale). | ||
** A Claviceps spore infects a flowering grass or cereal's floret. Upon connection to the vascular bundle, soft white sphacelia tissue develops. This hardens and dries into a sclerotium in the destroyed floret's husk. | ** A Claviceps spore infects a flowering grass or cereal's floret. Upon connection to the vascular bundle, soft white sphacelia tissue develops. This hardens and dries into a sclerotium in the destroyed floret's husk. | ||
** Alkaloids and lipids accumulate in the sclerotium. Dry, mature claviceps purpurea sclerotium consist of about 2% ergot alkaloids by weight. Claviceps africana also contains substantial alkaloids. | ** Alkaloids and lipids accumulate in the sclerotium. Dry, mature claviceps purpurea sclerotium consist of about 2% ergot alkaloids by weight. Claviceps africana also contains substantial ergot alkaloids. | ||
** Petri-grown Claviceps sees best results with potato dextrose or malt extract agar. | ** Petri-grown Claviceps sees best results with potato dextrose or malt extract agar. | ||
* '''Alkaloid''': Basic (high-pH) naturally-occurring organic compounds containing nitrogen. The "true alkaloids" are biosynthesized from amino acids and contain nitrogen in a heterocycle (a cyclic structure containing more than one element). | * '''Alkaloid''': Basic (high-pH) naturally-occurring organic compounds containing nitrogen. The "true alkaloids" are biosynthesized from amino acids and contain nitrogen in a heterocycle (a cyclic structure containing more than one element). | ||
** '''Indole''': The aromatic heterocycle C<sub>8</sub>H<sub>7</sub>N. Bicyclic pair of benzene (C<sub>6</sub>H<sub>6</sub>) and pyrrole (C<sub>4</sub>H<sub>4</sub>NH) sharing an edge. A biosynthetic precursor to the indole alkaloids, including the amino acid tryptophan and 3-indolepropionic acid (IPA). | ** '''Indole''': The aromatic heterocycle C<sub>8</sub>H<sub>7</sub>N. Bicyclic pair of benzene (C<sub>6</sub>H<sub>6</sub>) and pyrrole (C<sub>4</sub>H<sub>4</sub>NH) sharing an edge. A biosynthetic precursor to the indole alkaloids, including the amino acid tryptophan and the neuroprotective antioxidant 3-indolepropionic acid (IPA). | ||
** '''Tryptophan''': An essential amino acid (one which cannot be biosynthesized in humans), and a precursor in humans of serotonin, melatonin, and vitamin B3. Clostridium sporogenes in the human gut | ** '''Tryptophan''': An essential amino acid (one which cannot be biosynthesized in humans), and a precursor in humans of serotonin, melatonin, and vitamin B3. Clostridium sporogenes in the human gut metabolize tryptophan into indole. | ||
** '''Ergoline''': the tetracyclic structural skeleton (C<sub>14</sub>H<sub>16</sub>N<sub>2</sub>) shared by ergoline alkaloids. Also a [https://jkproducts.us/ergoline-tanning-beds/ tanning bed] by JK Products (pronounced differently). | ** '''Ergoline''': the tetracyclic structural skeleton (C<sub>14</sub>H<sub>16</sub>N<sub>2</sub>) shared by ergoline alkaloids. Also a [https://jkproducts.us/ergoline-tanning-beds/ tanning bed] by JK Products (pronounced differently). | ||
<gallery> | |||
File:Agroclavine.png|Agroclavine|alt=agroclavine | |||
File:Elymoclavine.png|Elymoclavine|alt=Elymoclavine | |||
File:Paspalic.png|Paspalic acid|alt=Paspalic acid | |||
File:DLA.png|DLA|alt=D-lysergic acid | |||
File:LSA.png|LSA|alt=D-lysergamide acid amide | |||
File:Lsd.png|LSD|alt=D-lysergic acid diethylamide | |||
</gallery> | |||
==Ergoline path (biosynthesis of the ergot alkaloids)== | ==Ergoline path (biosynthesis of the ergot alkaloids)== | ||
[[File:Biosynthesis-dla.png|right|thumb|Orange indicates alternate branch paths. Green is common to all ergot species, blue is species-specific.]] | [[File:Biosynthesis-dla.png|right|thumb|Orange indicates alternate branch paths. Green is common to all ergot species, blue is species-specific.]] | ||
The road to | The biological road to psychedelic ergolines begins with L-tryptophan and culminates in D-lysergic acid. The ergoline skeleton is complete in agroclavine (and indeed apparent in its three sibling daughters of chanoclavine). Ergolines are generally classified as one of: | ||
* Clavines (not generally psychoactive) | * Clavines (not generally psychoactive) | ||
* Ergopeptines (ergopeptides). Water-insoluble. | * Ergopeptines (ergopeptides). Water-insoluble. | ||
| Line 25: | Line 34: | ||
** Ergine (LSA, D-lysergic acid amide) | ** Ergine (LSA, D-lysergic acid amide) | ||
** LSD-25 (LSD, D-lysergic acid diethylamide) | ** LSD-25 (LSD, D-lysergic acid diethylamide) | ||
Note that ergine occurs naturally in some vines of the Convolvulaceae family (Hawaiian baby rosebud aka Argyreia nervosa, morning glory aka Ipomoea tricolor, and ololiúqui aka Ipomoea corymbosa), and is thus an ergoline alkaloid. There is no known natural source of | Should one's interests lie in the lysergamides, ergopeptides are '''not''' a necessary precursor, but perhaps more conveniently acquired than lysergic acid (any preparation of ergot fungus will furthermore require hydrolysis of its accumulated alkaloids). Note that ergine occurs naturally in some vines of the Convolvulaceae family (Hawaiian baby rosebud aka Argyreia nervosa, morning glory aka Ipomoea tricolor, and ololiúqui aka Ipomoea corymbosa), and is thus an ergoline alkaloid. There is no known natural source of LSD-25. | ||
LSA is a mild psychedelic. | |||
LSD is just about the most potent psychedelic by weight known to man. | |||
* L- | * L-tryptophan (C<sub>11</sub>H<sub>12</sub>N<sub>2</sub>O<sub>2</sub>) -> (prenylation via DMAPP from mevalonic acid, catalyzed by DMATS) | ||
** DMAT(S): dimethylallyltryptophan (synthetase) | ** DMAT(S): dimethylallyltryptophan (synthetase) | ||
* 4-L-DMAT -> (N-methylation via SAM, catalyzed by EasF) | * 4-L-DMAT -> (N-methylation via SAM, catalyzed by EasF) | ||
* 4-DMA-L-abrine -> (decarboxylation+oxidation via EasC, catalyzed by EasE) | * 4-DMA-L-abrine -> (decarboxylation+oxidation via EasC, catalyzed by EasE) | ||
* Chanoclavine-I -> (oxidation via EasD) | * Chanoclavine-I (C<sub>16</sub>H<sub>20</sub>N<sub>2</sub>O) -> (oxidation via EasD) | ||
* Chanoclavine-I-aldehyde (a [https://en.wikipedia.org/wiki/Dopamine_receptor_D2 D<sub>2</sub> dopamine receptor] stimulant) -> | * Chanoclavine-I-aldehyde (a [https://en.wikipedia.org/wiki/Dopamine_receptor_D2 D<sub>2</sub> dopamine receptor] stimulant) -> | ||
* Agroclavine -> | * Agroclavine (C<sub>16</sub>H<sub>18</sub>N<sub>2</sub>)-> | ||
* D- | ** Alternate branch: Elymoclavine (C<sub>16</sub>H<sub>18</sub>N<sub>2</sub>O) -> | ||
** Paspalic acid (C<sub>16</sub>H<sub>16</sub>N<sub>2</sub>O<sub>2</sub>)-> (see below) | |||
* D-lysergic acid (DLA, C<sub>16</sub>H<sub>16</sub>N<sub>2</sub>O<sub>2</sub>). | |||
Chanoclavine-I-aldehyde branches to festuclavine, pyroclavine, and cycloclavine (in addition to agroclavine), leading to the dihydroergopeptides and fumigaclavines. In addition to DLA, agroclavine branches to emiloclavine (also seen as emyloclavine) and thus paspalic acid. | Chanoclavine-I-aldehyde branches to festuclavine, pyroclavine, and cycloclavine (in addition to agroclavine), leading to the dihydroergopeptides and fumigaclavines. In addition to DLA, agroclavine branches to emiloclavine (also seen as emyloclavine) and thus paspalic acid. | ||
===Paspalic acid=== | ===Paspalic acid=== | ||
Paspalic acid is an isomer of lysergic acid, and will spontaneously convert. | Paspalic acid is an isomer of lysergic acid, and will spontaneously convert. Jean-Claude Gaullier's 2000 [https://patents.google.com/patent/US6242603B1/en patent] specifies a high-yield (81.6% RRi, 2.8% iso-LA) isomerization using a tetra(C1-C6)alkylammonium hydroxide. In that same patent, he cites previous isomerizations using 2N sodium hydroxide or 0.5N potassium hydroxide, reporting 59.3% RRi with NaOH (6.8% iso-LA) and 49.8% RRi with KOH (1% iso-LA), described in the Swiss journal <i>Helvetica Chimica Acta</i> in 1981 and 1964, respectively. [https://patents.google.com/patent/WO2005082902A1/en Cvac + Mojczek claimed] to do even better purity-wise in 2005 by following the metallic hydroxide with a methanol wash. | ||
===Extraction of ergopeptines from ergot broth=== | |||
Stoll patented the isolation of ergotamine tartrate in 1918 at Sandoz. | |||
'''WRITEME''' | |||
===Bioengineered ergolines=== | |||
'''WRITEME''' provide details of gene coding for enzymes | |||
==D-lysergic acid== | ==D-lysergic acid== | ||
Lysergic: discovered via hydro''lys''is of ''erg''otamine | [[File:Lysergamide.png|right|thumb|Functional diagram of substituted ergines (see table).]] | ||
Lysergic: discovered via hydro''lys''is of ''erg''otamine (-ic: a suffix meaning "characterized by"). | |||
'''The ergopeptides are biosynthetic derivatives of DLA, but DLA is a synthetic derivative of the ergopeptides.''' DLA was first isolated by hydrolysis of ergot alkaloids in the 1930s (Jacobs & Craig, [https://www.sciencedirect.com/science/article/pii/S0021925818757293 "The Ergot Alkaloids: II. The Degradation of Ergotinine with Alkali. Lysergic Acid"], ''Journal of Biological Chemistry'' 1934). It was first synthesized by Woodward in 1956 starting from 3ß-carboxyethylindole, and numerous processes have been published since: see [https://www.mdpi.com/1420-3049/27/21/7322 this 2022 review] for full details. The manufacture of LSD requires DLA, which has been historically hydrolyzed from bulk ergot alkaloids due to the difficulty of | A Table I precursor under the [https://en.wikisource.org/wiki/United_Nations_Convention_Against_Illicit_Traffic_in_Narcotic_Drugs_and_Psychotropic_Substances UN Convention Against Psychotropic Substances] and a Schedule III controlled substance. About 15 tons of DLA (also seen as (+)-lysergic acid) are legitimately manufactured each year, primarily from submerged fermentation of special strains of Calviceps purpurea, but also from field harvests wherever eastern europeans can be convinced to tromp around filling their baskets with mycotoxic jizz. | ||
{| class="wikitable" | |||
|+ Substituted lysergamides (see diagram on right) | |||
|- | |||
!Name !! R<sup>1</sup> !! R<sup>2</sup> !! R<sup>3</sup> | |||
|- | |||
| Ergine || H || H || H | |||
|- | |||
| Ergonovine || H || CH(CH<sub>3</sub>)CH<sub>2</sub>OH || H | |||
|- | |||
| Methergine || H || CH(CH<sub>2</sub>CH<sub>3</sub>)CH<sub>2</sub>OH || H | |||
|- | |||
| Methysergide || CH<sub>3</sub> (methyl) || CH(CH<sub>2</sub>CH<sub>3</sub>)CH<sub>2</sub>OH || H | |||
|- | |||
| LSD || H || CH<sub>2</sub>CH<sub>3</sub> (ethyl group) || CH<sub>2</sub>CH<sub>3</sub> | |||
|} | |||
'''The ergopeptides are biosynthetic derivatives of DLA, but DLA is a synthetic derivative of the ergopeptides.''' DLA was first isolated by hydrolysis of ergot alkaloids in the 1930s (Jacobs & Craig, [https://www.sciencedirect.com/science/article/pii/S0021925818757293 "The Ergot Alkaloids: II. The Degradation of Ergotinine with Alkali. Lysergic Acid"], ''Journal of Biological Chemistry'' 1934). It was first synthesized by Woodward's team in 1956 starting from 3ß-carboxyethylindole (Kornfield et al, [https://pubs.acs.org/doi/pdf/10.1021/ja01594a039 "The Total Synthesis of Lysergic Acid"], ''Journal of the American Chemical Society'' 1956-07), and numerous processes have been published since: see [https://www.mdpi.com/1420-3049/27/21/7322 this 2022 review] for full details. The manufacture of LSD requires DLA, which has been historically hydrolyzed from bulk ergot alkaloids due to the difficulty of total syntheses. Controlled biosynthesis ought cease at DLA; should you have the opportunity to acquire DLA, you're ready to go to town and/or prison. | |||
===Stereoisomers=== | ===Stereoisomers=== | ||
'''WRITEME''' | |||
===Production from agroclavine=== | |||
'''WRITEME''' | |||
===Production from ergopeptides=== | |||
'''WRITEME''' | |||
==D-lysergic acid diethylamide== | ==D-lysergic acid diethylamide== | ||
Hoffman first synthesized LSD ("Lysergsäurediethylamid") 1938-11-16 at Sandoz Pharmaceuticals. On 1943-04-19, he accidentally ingested about 250 micrograms, and discovered its potent effects. | [[File:Lysergsäurediethylamid.png|right|thumb|One of the more interesting semisynthetic ergolines.]] | ||
(6aR,9R)-N,N-diethyl-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide is a guaranteed cure for boredom. | |||
Hoffman first synthesized LSD ("Lysergsäurediethylamid", C<sub>20</sub>H<sub>25</sub>N<sub>3</sub>O) 1938-11-16 at Sandoz Pharmaceuticals. It was the twenty-fifth substance synthesized in a program of systematically exploring the ergot alkaloids, hence the name LSD-25. On 1943-04-19, he accidentally ingested about 250 micrograms, and discovered its potent effects. Some claim this to be evidence of God's direct intervention in human affairs, but I am unconvinced. | |||
===Production from DLA=== | |||
'''WRITEME''' | |||
====Synthesis of CDI==== | |||
N,N'-Carbonyldiimidazole (CDI) is a fine activating agent for DLA, but if it is inaccessible, it can be synthesized from imidazole and phosgene. Lysergic acid hydrate must be dried before activation with CDI! '''WRITEME''' | |||
====Drying DLA hydrate==== | |||
'''WRITEME''' | |||
===Production from other lysergamides=== | |||
No cost-effective means is known by which substantial LSD can be produced from organic material containing LSA (particularly the Convolvulaceae vines mentioned earlier). Should one have a supply of pure ergine, that's a different story. '''WRITEME''' | |||
== | ===Conversion from stereoisomers=== | ||
'''WRITEME''' | |||
== | ===Pharmacology=== | ||
'''WRITEME''' | |||
===Testing=== | |||
UV flourescence spectroscopy will destroy the sample. | |||
The Ehrlich reagent is sensitive to indole alkaloids, turning purple or dark pink. This is qualitative (basically "present" or "not present"), has many false positives (pyridoxine, other tryptamines...), and can't speak to purity. If it fails, however, you almost certainly have no ergoline, and will not be going to space today. The Marquis reagent ought turn "olive black", whatever that is. The Marquis reagent is [https://www.erowid.org/columns/crew/2015/06/marquis-and-lsd-is-color-change-visible/ not considered a good test for LSD]. | |||
* NMR '''WRITEME''' | |||
====Mass spectrometry==== | |||
===Practical matters=== | |||
'''WRITEME''' | |||
==LSD derivatives== | |||
* 1P-LSD '''WRITEME''' | |||
* ALD-52 '''WRITEME''' | |||
* LSZ (Lysergic acid 2,4-dimethylazetidide) aka λ '''WRITEME''' | |||
* ETH-LAD (see below) | |||
==Experimental== | |||
Some things I'm not yet sure about. | |||
What were Hoffman's first 24 ergot derivatives? It's unlikely that there are any lost treasures among them—Hoffman was perhaps the world's foremost authority on ergot chemistry, and spent much of his life doing psychedelic research. | |||
===Back-synthesis from DLA derivatives=== | |||
Diversion-o-clock! Remember those 15 tons of legitimately-produced lysergic acid? That's ''in addition to'' about ten tons of ergotamine alkaloids; i.e. your Cafergot (ergotamine tartrate + caffeine) and Syntometrine (ergometrine + oxytocin) aren't counting against that total. So there are about 25 tons of pharmaceutical precursor being generated each year. Where is it all going? Is it going anywhere where we can get it back? | |||
* Sermion (nicergoline) | |||
* Parlodel (bromocriptine, ergocryptine derivative) | |||
* ... | |||
Can we do anything with ergosterol (available from lanosterol)? | |||
===Chasing perf=== | |||
Can we improve upon LSD's binding efficiencies? Can we do so cost-effectively? | |||
* ETH-LAD '''WRITEME''' | |||
==tl;dr== | |||
My opinion is that modern LSD manufacture, barring connections to the Chinese or Indian underworlds or a few acres of toxified rye, is best accomplished via controlled biosynthesis of DLA in yeasts followed by DLA conversion in a microreactor array. Extraction and hydrolysis of alkaloids from a broth of petri-grown ergot is also completely feasible, and easily scaled to kilograms. You do not need a megagram of LSD (you probably don't need a kilogram). Either way, the chemistry is not complex, but it does require precision, patience, a solid few days without interruption, and a significant investment in equipment. Making effective use of precursors requires multiple rounds, as does purification of the single psychoactive stereoisomer. Performing these tedious rounds while avoiding accidental exposure is perhaps the most difficult practical aspect of manufacture. | |||
Isolsd and lumalsd are not psychoactive, but seem to put equal stress on the body otherwise. Raising the unit dosage to compensate for an impure result will deliver more of these undesirable contaminants. Good product '''requires repeated conversion of stereoisomers'''. | |||
Distribution/manufacture/possession with intent of 1g LSD is a five-year mandatory minimum under 21 USC 841(b)(1)(B). Ten grams is a ten year mandatory minimum under 21 USC 841(b)(1)(A). Be careful out there. Attempting to buy controlled precursors will almost certainly land you in prison: do not trust Greeks bearing kilograms of ergotamine tartrate. | |||
Don't eat ergot. | |||
==External links== | ==External links== | ||
I'm not going to link to all the various papers and patents for synthesizing DLA, as they're well-covered by review articles. | I'm not going to link to all the various papers and patents for synthesizing DLA, as they're well-covered by review articles. Fukuyama et al's [https://pubs.acs.org/doi/pdf/10.1021/ol4019562 Total Synthesis of Lysergic Acid] (''Organic Letters'' 2013) seems pretty much the state of the art for abiological methods (note that this paper had a correction issued in 2014, which does not affect the results). | ||
* [https://www.mdpi.com/1420-3049/27/21/7322 Methods of Lysergic Acid Synthesis]. Jastrzębski et al. ''Molecules'' 2022-10. | * [https://www.mdpi.com/1420-3049/27/21/7322 Methods of Lysergic Acid Synthesis]. Jastrzębski et al. ''Molecules'' 2022-10. | ||
* [https://pubmed.ncbi.nlm.nih.gov/35132076/ Reconstituting the complete biosynthesis of D-lysergic acid in yeast]. Wong et al. ''Nature Communications'' 2022-02. | * [https://pubmed.ncbi.nlm.nih.gov/35132076/ Reconstituting the complete biosynthesis of D-lysergic acid in yeast]. Wong et al. ''Nature Communications'' 2022-02. | ||
* [https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Complex_Molecular_Synthesis_(Salomon)/06%3A_Amino_Acids_and_Alkaloids/6.04%3A_Lysergic_Acid Biosynthesis of Tryptophan and Lysergic Acid]. Salomon. ''Complex Molecular Synthesis'' LibreTexts Chemistry 2021. | |||
* [https://www.sciencedirect.com/science/article/abs/pii/S1099483120300298 Biosynthesis, total synthesis, and biological profiles of ergot alkaloids]. Tasker & Wipf. ''The Alkaloids: Chemistry and Biology'' 2020. | * [https://www.sciencedirect.com/science/article/abs/pii/S1099483120300298 Biosynthesis, total synthesis, and biological profiles of ergot alkaloids]. Tasker & Wipf. ''The Alkaloids: Chemistry and Biology'' 2020. | ||
* [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4829483/ Return of the Lysergamides] Parts I-VII. Brandt et al. ''Drug Testing and Analysis'' 2016-09. | |||
* [https://sci-hub.se/10.1023/A:1014050926916 The Chemistry of Peptide Ergot Alkaloids]. Komarova & Tolkachev. ''Pharmaceutical Chemistry Journal'' 2001-02, translated 2001-09. | * [https://sci-hub.se/10.1023/A:1014050926916 The Chemistry of Peptide Ergot Alkaloids]. Komarova & Tolkachev. ''Pharmaceutical Chemistry Journal'' 2001-02, translated 2001-09. | ||
* Lysergamides Revisited. Nichols et al. ''Hallucinogens: An Update''. National Institute on Drug Abuse Research Monograph Series #146 1994. | |||
* [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC546981/ Ergotamine Production in Submerged Culture and Physiology of Claviceps purpurea]. Amici et al. ''Applied Microbiology'' 1967-05. | * [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC546981/ Ergotamine Production in Submerged Culture and Physiology of Claviceps purpurea]. Amici et al. ''Applied Microbiology'' 1967-05. | ||
* [https://www.incb.org/documents/PRECURSORS/RED_LIST/Red_List_2022_19th_edition_E.pdf List of Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances under International Control]. International Narcotics Control Board 2022-01. Also known as the "Red List". | * [https://www.incb.org/documents/PRECURSORS/RED_LIST/Red_List_2022_19th_edition_E.pdf List of Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances under International Control]. International Narcotics Control Board 2022-01. Also known as the "Red List". | ||
* [http://www.psychedelic-library.org/books/Kosmos+Cover.pdf Chapter 10, "Chemistry"]. Webster. ''Kosmos: A Theory of Psychedelic Experience'' (unpublished 2001 draft). | |||
* [https://the-hive.archive.erowid.org/forum/threads.pl?Cat=&Board=tryptamine Tryptamine and Ergoline Chemistry] on the Hive (archived at Erowid) | * [https://the-hive.archive.erowid.org/forum/threads.pl?Cat=&Board=tryptamine Tryptamine and Ergoline Chemistry] on the Hive (archived at Erowid) | ||
* ''[https://erowid.org/library/books_online/tihkal/tihkal.shtml Tryptamines i Have Known And Loved: The Continuation]''. Shulgin & Shulgin. Transform Press 1997. | |||
Anything [https://www.researchgate.net/profile/David-Nichols-9 David Nichols] publishes is worth reading. | |||
Latest revision as of 03:25, 9 August 2023
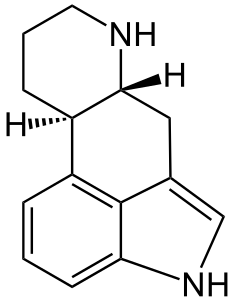
Ergot is pronounced ur-git, not er-go. Highly toxic, it ought not be consumed. Properly modified, it has many uses, primarily due to fortuitous similarity between the ergoline skeleton and the monoamide neurotransmitters (e.g. serotonin, dopamine, noradrenaline)...
Ergot alkaloids ergonovine and ergotamine (and their salts) are both original List I controlled precursors under the 1970 Controlled Substances Act. Ergocristine was added to List I in 2010 (see the DEA's 2010-03 Microgram Journal). Derivatives lysergic acid and its amide ("ergine") are Schedule III controlled substances under the CSA, and ergine is a Class A precursor in the United Kingdom. Subsequent derivative lysergic acid diethylamide is an original Schedule I controlled substance, and its functional analogues (any chemical "substantially similar", as if that had any kind of precise meaning) are treated as Schedule I due to the 1986 Federal Analogue Act. Ergotamine, lysergic acid, and ergometrine (ergonovine) are all on Table I of the INCB Red List, and are thus EU Category 1 precursors. So there's clearly something worth knowing about ergot!
Some basic terminology
- Ergot: fungi of the genus Claviceps. All Claviceps species are ergot. The most well-known member is Claviceps purpurea (Latin purpuro, purple, "to adorn/beautify"), the rye ergot fungus, which is parasitic on grasses and cereals (especially rye, Secale cereale).
- A Claviceps spore infects a flowering grass or cereal's floret. Upon connection to the vascular bundle, soft white sphacelia tissue develops. This hardens and dries into a sclerotium in the destroyed floret's husk.
- Alkaloids and lipids accumulate in the sclerotium. Dry, mature claviceps purpurea sclerotium consist of about 2% ergot alkaloids by weight. Claviceps africana also contains substantial ergot alkaloids.
- Petri-grown Claviceps sees best results with potato dextrose or malt extract agar.
- Alkaloid: Basic (high-pH) naturally-occurring organic compounds containing nitrogen. The "true alkaloids" are biosynthesized from amino acids and contain nitrogen in a heterocycle (a cyclic structure containing more than one element).
- Indole: The aromatic heterocycle C8H7N. Bicyclic pair of benzene (C6H6) and pyrrole (C4H4NH) sharing an edge. A biosynthetic precursor to the indole alkaloids, including the amino acid tryptophan and the neuroprotective antioxidant 3-indolepropionic acid (IPA).
- Tryptophan: An essential amino acid (one which cannot be biosynthesized in humans), and a precursor in humans of serotonin, melatonin, and vitamin B3. Clostridium sporogenes in the human gut metabolize tryptophan into indole.
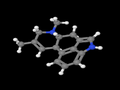
- Ergoline: the tetracyclic structural skeleton (C14H16N2) shared by ergoline alkaloids. Also a tanning bed by JK Products (pronounced differently).
-
Agroclavine
-
Elymoclavine
-
Paspalic acid
-
DLA
-
LSA
-
LSD
Ergoline path (biosynthesis of the ergot alkaloids)
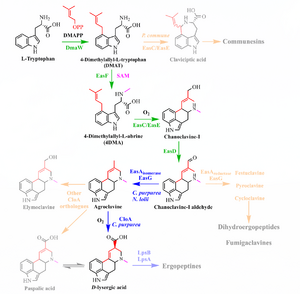
The biological road to psychedelic ergolines begins with L-tryptophan and culminates in D-lysergic acid. The ergoline skeleton is complete in agroclavine (and indeed apparent in its three sibling daughters of chanoclavine). Ergolines are generally classified as one of:
- Clavines (not generally psychoactive)
- Ergopeptines (ergopeptides). Water-insoluble.
- Ergotoxines: ergocristine, ergocornine, ɑ- and β-ergocryptine
- Ergotamine group: ergotamine, ergovaline, ɑ- and β-ergosine
- Lysergamides (lysergic acid amides). Water-soluble. Of particular interest are:
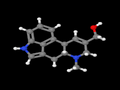
- Ergine (LSA, D-lysergic acid amide)
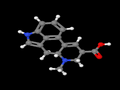
- LSD-25 (LSD, D-lysergic acid diethylamide)
Should one's interests lie in the lysergamides, ergopeptides are not a necessary precursor, but perhaps more conveniently acquired than lysergic acid (any preparation of ergot fungus will furthermore require hydrolysis of its accumulated alkaloids). Note that ergine occurs naturally in some vines of the Convolvulaceae family (Hawaiian baby rosebud aka Argyreia nervosa, morning glory aka Ipomoea tricolor, and ololiúqui aka Ipomoea corymbosa), and is thus an ergoline alkaloid. There is no known natural source of LSD-25.
LSA is a mild psychedelic. LSD is just about the most potent psychedelic by weight known to man.
- L-tryptophan (C11H12N2O2) -> (prenylation via DMAPP from mevalonic acid, catalyzed by DMATS)
- DMAT(S): dimethylallyltryptophan (synthetase)
- 4-L-DMAT -> (N-methylation via SAM, catalyzed by EasF)
- 4-DMA-L-abrine -> (decarboxylation+oxidation via EasC, catalyzed by EasE)
- Chanoclavine-I (C16H20N2O) -> (oxidation via EasD)
- Chanoclavine-I-aldehyde (a D2 dopamine receptor stimulant) ->
- Agroclavine (C16H18N2)->
- Alternate branch: Elymoclavine (C16H18N2O) ->
- Paspalic acid (C16H16N2O2)-> (see below)
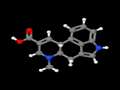
- D-lysergic acid (DLA, C16H16N2O2).
Chanoclavine-I-aldehyde branches to festuclavine, pyroclavine, and cycloclavine (in addition to agroclavine), leading to the dihydroergopeptides and fumigaclavines. In addition to DLA, agroclavine branches to emiloclavine (also seen as emyloclavine) and thus paspalic acid.
Paspalic acid
Paspalic acid is an isomer of lysergic acid, and will spontaneously convert. Jean-Claude Gaullier's 2000 patent specifies a high-yield (81.6% RRi, 2.8% iso-LA) isomerization using a tetra(C1-C6)alkylammonium hydroxide. In that same patent, he cites previous isomerizations using 2N sodium hydroxide or 0.5N potassium hydroxide, reporting 59.3% RRi with NaOH (6.8% iso-LA) and 49.8% RRi with KOH (1% iso-LA), described in the Swiss journal Helvetica Chimica Acta in 1981 and 1964, respectively. Cvac + Mojczek claimed to do even better purity-wise in 2005 by following the metallic hydroxide with a methanol wash.
Extraction of ergopeptines from ergot broth
Stoll patented the isolation of ergotamine tartrate in 1918 at Sandoz. WRITEME
Bioengineered ergolines
WRITEME provide details of gene coding for enzymes
D-lysergic acid
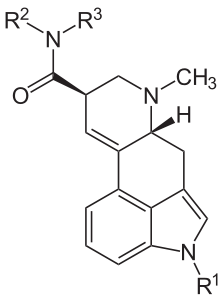
Lysergic: discovered via hydrolysis of ergotamine (-ic: a suffix meaning "characterized by").
A Table I precursor under the UN Convention Against Psychotropic Substances and a Schedule III controlled substance. About 15 tons of DLA (also seen as (+)-lysergic acid) are legitimately manufactured each year, primarily from submerged fermentation of special strains of Calviceps purpurea, but also from field harvests wherever eastern europeans can be convinced to tromp around filling their baskets with mycotoxic jizz.
| Name | R1 | R2 | R3 |
|---|---|---|---|
| Ergine | H | H | H |
| Ergonovine | H | CH(CH3)CH2OH | H |
| Methergine | H | CH(CH2CH3)CH2OH | H |
| Methysergide | CH3 (methyl) | CH(CH2CH3)CH2OH | H |
| LSD | H | CH2CH3 (ethyl group) | CH2CH3 |
The ergopeptides are biosynthetic derivatives of DLA, but DLA is a synthetic derivative of the ergopeptides. DLA was first isolated by hydrolysis of ergot alkaloids in the 1930s (Jacobs & Craig, "The Ergot Alkaloids: II. The Degradation of Ergotinine with Alkali. Lysergic Acid", Journal of Biological Chemistry 1934). It was first synthesized by Woodward's team in 1956 starting from 3ß-carboxyethylindole (Kornfield et al, "The Total Synthesis of Lysergic Acid", Journal of the American Chemical Society 1956-07), and numerous processes have been published since: see this 2022 review for full details. The manufacture of LSD requires DLA, which has been historically hydrolyzed from bulk ergot alkaloids due to the difficulty of total syntheses. Controlled biosynthesis ought cease at DLA; should you have the opportunity to acquire DLA, you're ready to go to town and/or prison.
Stereoisomers
WRITEME
Production from agroclavine
WRITEME
Production from ergopeptides
WRITEME
D-lysergic acid diethylamide
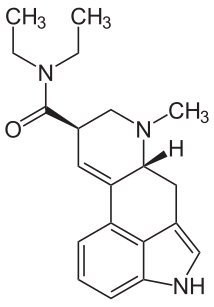
(6aR,9R)-N,N-diethyl-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide is a guaranteed cure for boredom.
Hoffman first synthesized LSD ("Lysergsäurediethylamid", C20H25N3O) 1938-11-16 at Sandoz Pharmaceuticals. It was the twenty-fifth substance synthesized in a program of systematically exploring the ergot alkaloids, hence the name LSD-25. On 1943-04-19, he accidentally ingested about 250 micrograms, and discovered its potent effects. Some claim this to be evidence of God's direct intervention in human affairs, but I am unconvinced.
Production from DLA
WRITEME
Synthesis of CDI
N,N'-Carbonyldiimidazole (CDI) is a fine activating agent for DLA, but if it is inaccessible, it can be synthesized from imidazole and phosgene. Lysergic acid hydrate must be dried before activation with CDI! WRITEME
Drying DLA hydrate
WRITEME
Production from other lysergamides
No cost-effective means is known by which substantial LSD can be produced from organic material containing LSA (particularly the Convolvulaceae vines mentioned earlier). Should one have a supply of pure ergine, that's a different story. WRITEME
Conversion from stereoisomers
WRITEME
Pharmacology
WRITEME
Testing
UV flourescence spectroscopy will destroy the sample.
The Ehrlich reagent is sensitive to indole alkaloids, turning purple or dark pink. This is qualitative (basically "present" or "not present"), has many false positives (pyridoxine, other tryptamines...), and can't speak to purity. If it fails, however, you almost certainly have no ergoline, and will not be going to space today. The Marquis reagent ought turn "olive black", whatever that is. The Marquis reagent is not considered a good test for LSD.
- NMR WRITEME
Mass spectrometry
Practical matters
WRITEME
LSD derivatives
- 1P-LSD WRITEME
- ALD-52 WRITEME
- LSZ (Lysergic acid 2,4-dimethylazetidide) aka λ WRITEME
- ETH-LAD (see below)
Experimental
Some things I'm not yet sure about.
What were Hoffman's first 24 ergot derivatives? It's unlikely that there are any lost treasures among them—Hoffman was perhaps the world's foremost authority on ergot chemistry, and spent much of his life doing psychedelic research.
Back-synthesis from DLA derivatives
Diversion-o-clock! Remember those 15 tons of legitimately-produced lysergic acid? That's in addition to about ten tons of ergotamine alkaloids; i.e. your Cafergot (ergotamine tartrate + caffeine) and Syntometrine (ergometrine + oxytocin) aren't counting against that total. So there are about 25 tons of pharmaceutical precursor being generated each year. Where is it all going? Is it going anywhere where we can get it back?
- Sermion (nicergoline)
- Parlodel (bromocriptine, ergocryptine derivative)
- ...
Can we do anything with ergosterol (available from lanosterol)?
Chasing perf
Can we improve upon LSD's binding efficiencies? Can we do so cost-effectively?
- ETH-LAD WRITEME
tl;dr
My opinion is that modern LSD manufacture, barring connections to the Chinese or Indian underworlds or a few acres of toxified rye, is best accomplished via controlled biosynthesis of DLA in yeasts followed by DLA conversion in a microreactor array. Extraction and hydrolysis of alkaloids from a broth of petri-grown ergot is also completely feasible, and easily scaled to kilograms. You do not need a megagram of LSD (you probably don't need a kilogram). Either way, the chemistry is not complex, but it does require precision, patience, a solid few days without interruption, and a significant investment in equipment. Making effective use of precursors requires multiple rounds, as does purification of the single psychoactive stereoisomer. Performing these tedious rounds while avoiding accidental exposure is perhaps the most difficult practical aspect of manufacture.
Isolsd and lumalsd are not psychoactive, but seem to put equal stress on the body otherwise. Raising the unit dosage to compensate for an impure result will deliver more of these undesirable contaminants. Good product requires repeated conversion of stereoisomers.
Distribution/manufacture/possession with intent of 1g LSD is a five-year mandatory minimum under 21 USC 841(b)(1)(B). Ten grams is a ten year mandatory minimum under 21 USC 841(b)(1)(A). Be careful out there. Attempting to buy controlled precursors will almost certainly land you in prison: do not trust Greeks bearing kilograms of ergotamine tartrate.
Don't eat ergot.
External links
I'm not going to link to all the various papers and patents for synthesizing DLA, as they're well-covered by review articles. Fukuyama et al's Total Synthesis of Lysergic Acid (Organic Letters 2013) seems pretty much the state of the art for abiological methods (note that this paper had a correction issued in 2014, which does not affect the results).
- Methods of Lysergic Acid Synthesis. Jastrzębski et al. Molecules 2022-10.
- Reconstituting the complete biosynthesis of D-lysergic acid in yeast. Wong et al. Nature Communications 2022-02.
- Biosynthesis of Tryptophan and Lysergic Acid. Salomon. Complex Molecular Synthesis LibreTexts Chemistry 2021.
- Biosynthesis, total synthesis, and biological profiles of ergot alkaloids. Tasker & Wipf. The Alkaloids: Chemistry and Biology 2020.
- Return of the Lysergamides Parts I-VII. Brandt et al. Drug Testing and Analysis 2016-09.
- The Chemistry of Peptide Ergot Alkaloids. Komarova & Tolkachev. Pharmaceutical Chemistry Journal 2001-02, translated 2001-09.
- Lysergamides Revisited. Nichols et al. Hallucinogens: An Update. National Institute on Drug Abuse Research Monograph Series #146 1994.
- Ergotamine Production in Submerged Culture and Physiology of Claviceps purpurea. Amici et al. Applied Microbiology 1967-05.
- List of Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances under International Control. International Narcotics Control Board 2022-01. Also known as the "Red List".
- Chapter 10, "Chemistry". Webster. Kosmos: A Theory of Psychedelic Experience (unpublished 2001 draft).
- Tryptamine and Ergoline Chemistry on the Hive (archived at Erowid)
- Tryptamines i Have Known And Loved: The Continuation. Shulgin & Shulgin. Transform Press 1997.
Anything David Nichols publishes is worth reading.