Check out my first novel, midnight's simulacra!
Ergot: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
[[File:Ergoline.png|right|thumb|The ergoline skeleton.]] | [[File:Ergoline.png|right|thumb|The ergoline skeleton looks not entirely unlike a cock-n-balls.]] | ||
Ergot is pronounced ur-git, '''not''' er-go. Highly toxic, it ought not be consumed. Properly modified, it has many uses, primarily due to fortuitous similarity between the ergoline skeleton and the monoamide neurotransmitters (e.g. serotonin, dopamine, adrenaline)... | Ergot is pronounced ur-git, '''not''' er-go. Highly toxic, it ought not be consumed. Properly modified, it has many uses, primarily due to fortuitous similarity between the ergoline skeleton and the monoamide neurotransmitters (e.g. serotonin, dopamine, adrenaline)... | ||
Revision as of 02:50, 3 January 2023
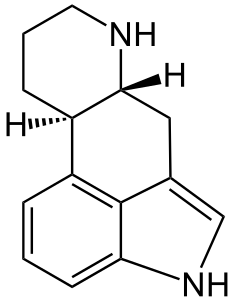
Ergot is pronounced ur-git, not er-go. Highly toxic, it ought not be consumed. Properly modified, it has many uses, primarily due to fortuitous similarity between the ergoline skeleton and the monoamide neurotransmitters (e.g. serotonin, dopamine, adrenaline)...
Some basic terminology
- Ergot: fungi of the genus Claviceps. All Claviceps species are ergot. The most well-known member is Claviceps purpurea (Latin purpuro, purple, "to adorn/beautify"), the rye ergot fungus, which is parasitic on grasses and cereals (especially rye, Secale cereale).
- A Claviceps spore infects a flowering grass or cereal's floret. Upon connection to the vascular bundle, soft white sphacelia tissue develops. This hardens and dries into a sclerotium in the destroyed floret's husk.
- Alkaloids and lipids accumulate in the sclerotium. Dry, mature claviceps purpurea sclerotium consist of about 2% ergot alkaloids by weight. Claviceps africana also contains substantial alkaloids.
- Alkaloid: Basic (high-pH) naturally-occurring organic compounds containing nitrogen. The "true alkaloids" are biosynthesized from amino acids and contain nitrogen in a heterocycle (a cyclic structure containing more than one element).
- Indole: The aromatic heterocycle C8H7N. Bicyclic pair of benzene (C6H6) and pyrrole (C4H4NH) sharing an edge. A biosynthetic precursor to the indole alkaloids, including the amino acid tryptophan and 3-indolepropionic acid (IPA).
- Tryptophan: An essential amino acid (one which cannot be biosynthesized in humans), and a precursor in humans of serotonin, melatonin, and vitamin B3. Clostridium sporogenes in the human gut metabolizes tryptophan into indole.
- Ergoline: the tetracyclic structural skeleton (C14H16N2) shared by ergoline alkaloids. Also a tanning bed by JK Products (pronounced differently).
Ergoline path (biosynthesis of the ergot alkaloids)
The road to the ergopeptides begins with L-tryptophan and culminates in D-lysergic acid. The ergoline skeleton is complete in agroclavine (and indeed apparent in its three sibling daughters of chanoclavine). Note that if one is interested in the water-soluble lysergamides, ergopeptines are not a necessary precursor.
- L-Tryptophan C11H12N2O2 -> (prenylation via DMAPP from mevalonic acid, catalyzed by DMATS)
- DMAT(S): dimethylallyltryptophan (synthetase)
- 4-L-DMAT -> (N-methylation via SAM, catalyzed by EasF)
- 4-DMA-L-abrine -> (decarboxylation+oxidation via EasC, catalyzed by EasE)
- Chanoclavine-I -> (oxidation via EasD)
- Chanoclavine-I-aldehyde (a D2 dopamine receptor stimulant) ->
- Agroclavine ->
- D-Lysergic acid (DLA).
Chanoclavine-I-aldehyde branches to festuclavine, pyroclavine, and cycloclavine (in addition to agroclavine), leading to the dihydroergopeptides and fumigaclavines. In addition to DLA, agroclavine branches to emiloclavine (also seen as emyloclavine) and thus paspalic acid.
Paspalic acid
Paspalic acid is an isomer of lysergic acid, and will spontaneously convert. Efficient isomerization can be achieved using 2N sodium hydroxide or 0.5N potassium hydroxide. In 1999, Jean-Claude Gaullier reported 59.3% RRi with NaOH (6.8% iso) and 49.8% RRi with KOH (1% iso).
D-Lysergic acid
Lysergic: discovered via hydrolysis of ergotamine. A Table I precursor under the UN Convention Against Psychotropic Substances. About 15 tons of DLA (also seen as (+)-lysergic acid) are legitimately manufactured each year, primarily from submerged fermentation of special strains of Calviceps purpurea but also from field harvests.
External links
- Reconstituting the complete biosynthesis of D-lysergic acid in yeast. Wong et al. Nature Communications 2022-02.
- Biosynthesis, total synthesis, and biological profiles of ergot alkaloids. Tasker & Wipf. The Alkaloids: Chemistry and Biology 2020.
- The Chemistry of Peptide Ergot Alkaloids. Komarova & Tolkachev. Pharmaceutical Chemistry Journal 2001-02, translated 2001-09.
- List of Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances under International Control. International Narcotics Control Board 2022-01. Also known as the "Red List".